If you are pursuing a career as a mammography technologist (mammographer) you should be familiar with the American College of Radiology (ACR) as a key professional medical society and body of clinical accreditation. But what does ACR mammography accreditation mean to a facility, and why should you care about it as a practicing or aspiring mammographer? Furthermore, what does the ACR accreditation process look like and what lies at its end? For answers to these questions and more, read on!
What is ACR accreditation in mammography?
First and foremost, ACR mammography accreditation ensures full patient safety and optimum service quality. As part of its focus on quality, the ACR has strict standards in place to optimize the production of clear and actionable diagnostic mammogram images. To meet these objectives, ACR officials must certify that each qualifying facility meets specific requirements in terms of medical imaging equipment, clinical personnel, and quality assurance.
ACR imaging reviews are conducted by expert radiologists and medical physicists. All ACR methods and procedures are thoroughly peer-reviewed and designed by knowledgeable radiologists and medical physicists.
ACR accreditation is a great way to assure patients that they are benefiting from high levels of safety and quality while they are receiving mammography services. This accreditation is also critical for facilities when it comes to complying with federal, state, and local governmental regulations and meeting the third-party payer criteria of most major healthcare insurance companies.
Is ACR accreditation required for mammography?
Since 1994, the Mammography Quality Standards Act (MQSA) has required all mammography facilities in the United States to secure and maintain official accreditation through an organization that is approved by the US Food and Drug Administration (FDA). And the only organization that currently has nationwide FDA approval in mammography accreditation is the American College of Radiology. This positions the ACR as an essential piece of the larger mammography industry puzzle.
How does a mammogram facility get accredited?
ACR mammography guidelines clearly detail the organization’s screening guidelines on everything from image quality to radiation dose. Through its Mammography Accreditation Program, it leads facilities through multiple stages of peer review while offering constructive feedback in areas that include equipment standards, staff qualifications, quality control, and quality assurance.
Leveraging the power of digital technology, the ACR has developed a streamlined accreditation process that enables them to complete evaluations in less than two months. The process begins when a facility submits an online application and pays the necessary application fee.
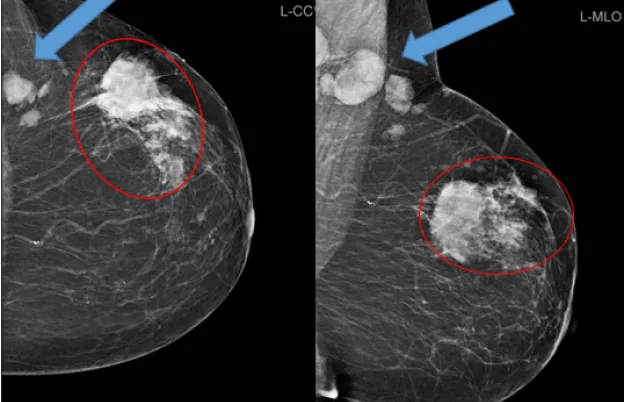
When the ACR accepts its application, the facility has 45 days to submit a testing package. This testing package must include an image of an ACR-approved phantom for review. A phantom is a standardized image that is commonly used as a quality control tool in the mammography industry. The ACR has posted its particular and specific phantom scoring and assessment standards on the Phantom Testing: Mammography page of its Accreditation Testing policies.
When it receives the testing packet, the ACR examines it internally before sending it to medical reviewers with the appropriate expertise to analyze its various components. After completing the review process, the ACR issues a final report back to the facility.
In some cases, facilities fail to achieve accreditation approval of their first testing package. When this occurs, the final report notes areas of concern and potential improvement. After working on these areas, facilities re-submit that part of their testing package with corrections. Once approved, facilities receive a 3-year certificate of accreditation. Before this accreditation terminates, the ACR invites facilities to submit a renewal application and begin the testing process once again.
How to address facility deficiencies during the ACR approval process
As detailed above, the ACR’s final report provides specific improvement recommendations for any facility that does not pass accreditation. The ACR statute Options Following a Failure or Deficiency: Mammography states, “After a failed outcome, the facility has the opportunity to submit an option form to proceed with accreditation through a repeat, reinstatement, appeal, or withdrawal of the unit in question from the accreditation process entirely.”
Facilities that choose to resubmit should address all improvement recommendations promptly and thoroughly. When a facility is deemed deficient on its first attempt at accreditation, the ACR simply advises it to undertake corrective action of its own accord. Subsequent ACR deficiencies, however, come with additional stipulations.
The ACR must notify the FDA about any facility that fails its second accreditation attempt, requiring that facility to submit appropriate documentation of each corrective measure that it took to achieve reinstatement. Any facility that receives a third deficiency must cease operations until it can complete a successful appeal. MTMI has resources to assist you. Contact us. We are here to help.
Build on ACR accreditation with the right continuing education
As we have already seen, technologist qualifications play an important part of the ACR accreditation process. This makes mammographers with the right training and continuing education extremely valuable to facilities across the United States. MTMI offers a wide variety of CE courses, allowing mammogram technologists and other imaging technologists (CT techs, MRI techs, Rad techs, US techs, OR techs, IR techs, PET/CT techs, Nuc Med techs) to receive relevant training and stay up to date on certification requirements from the comfort of their own homes.
MTMI programs are taught by experts with national reputations in their fields and cover every modality. Our cross-training courses, offered in classroom, via self-paced courses as well as via webinars, prepare you for registry exams and take your career to the next level.
For more information
Offering a range of customized consulting services, the Medical Technology Management Institute (MTMI) has helped countless clients with comprehensive ACR accreditation advisement. We tailor our consulting services to meet your specific needs, providing solutions that are directly applicable to your operations. While our expertise in Mammography and Breast Ultrasound is well known across the country, we want to highlight that our consulting services extend beyond these areas. Our team of experts is equipped to provide in-depth consulting services across various imaging modalities, including:
- Advanced techniques
- Regulatory compliance
- Workflow optimization
- MSK Ultrasound
- OB Ultrasound
- ECHO/Vascular Ultrasound
- PACS
- CT
- MRI
Check out our full catalog of programs or contact us with MQSA or ACR questions today!